Science has not been able to blame the protein gluten for all of the detrimental symptoms experienced by those with gluten sensitivity. It is common scientific knowledge that grain research is in its infancy. On that note, we discuss the lectin compound – wheat germ agglutinin (WGA)…
Science has not been able to blame the protein gluten for all of the detrimental symptoms experienced by those with gluten sensitivity. It is common scientific knowledge that grain research is in its infancy. On that note, we discuss the lectin compound – wheat germ agglutinin (WGA)…
The following was written by nutrition educator – Sayer Ji www.greenmedinfo.com Sayer does a fantastic job describing the effects of WGA on human health…
Below the radar of conventional serological testing for antibodies against the various gluten proteins and genetic testing for disease susceptibility, the WGA “lectin problem” remains almost entirely obscured. Lectins, though found in all grains, seeds, legumes, dairy and our beloved nightshades: the tomato and potato, are rarely discussed in connection with health or illness, even when their presence in our diet may greatly reduce both the quality and length of our lives.
Nature engineers, within all species, a set of defenses against predation, though not all are as obvious as the thorns on a rose or the horns on a rhinoceros. Plants do not have the cell-mediated immunity of higher life forms, like ants, nor do they have the antibody driven, secondary immune systems of vertebrates with jaws. They must rely on a much simpler, innate immunity. It is for this reason that seeds of the grass family, e.g. rice, wheat, spelt, rye, have exceptionally high levels of defensive glycoproteins known as lectins. Cooking, sprouting, fermentation and digestion are the traditional ways in which man, for instance, deals with the various anti-nutrients found within this family of plants, but lectins are, by design, particularly resistant to degradation through a wide range of pH and temperatures.
Lectins are glycoproteins, and through thousands of years of selectively breeding wheat for increasingly larger quantities of protein, the concentration of WGA lectin has increased proportionately. This, no doubt, has contributed to wheat’s global dominance as one of the world’s favored monocultures, offering additional “built-in” pest resistance. The word lectin comes from the same etymological root as the word select, and literally means “to choose.” Lectins are designed “to choose” specific carbohydrates that project off the surface of cells and upon which they attach. In the case of WGA the two glycoproteins it selects for, in order of greatest affinity, are N-Acetyl Glucosamine and N-Acetylneuraminic acid (sialic acid).
WGA is Nature’s ingenious solution for protecting the wheat plant from the entire gamut of its natural enemies. Fungi have cell walls composed of a polymer of N-Acetylglucosamine. The cellular walls of bacteria are made from a layered structure called the peptidoglycan, a biopolymer of N-Acetylglucosamine. N-acetylglucosamine is the basic unit of the biopolymer chitin, which forms the outer coverings of insects and crustaceans (shrimp, crab, etc.). All animals, including worms, fish, birds and humans, use N-Acetyglucosamine as a foundational substance for building the various tissues in their bodies, including the bones. The production of cartilage, tendons, and joints depend on the structural integrity of N-Acetylglucosamine. The mucous known as the glycocalyx, or literally, “sugar coat” is secreted in humans by the epithelial cells which line all the mucous membranes, from nasal cavities to the top to the bottom of the alimentary tube, as well as the protective and slippery lining of our blood vessels. The glycocalyx is composed largely of N-Acetylglucosamine and N-Acetylneuraminic acid (also known as sialic acid), with carbohydrate end of N-Acetylneuraminic acid of this protective glycoprotein forming the terminal sugar that is exposed to the contents of both the gut and the arterial lumen (opening). WGA’s unique binding specificity to these exact two glycoproteins is not accidental. Nature has designed WGA perfectly to attach to, disrupt, and gain entry through these mucosal surfaces.
WGA is most concentrated in the seed of the wheat plant, likely due to the fact that the seeds are the “babies” of these plants and are invested with the entire hope for continuance of their species. Protecting the seed against predation is necessarily a first priority. WGA is an exceedingly small glycoprotein (36 kilodaltons) and is concentrated deep within the embryo of the wheat berry (approximately 1 microgram per grain). WGA migrates during germination to the roots and tips of leaves, as the developing plant begins to project itself into the world and outside the safety of its seed. In its quest for nourishment from the soil, its roots are challenged with fungi and bacteria that seek to invade the plant. In its quest for sunlight and other nourishment from the heavens the plant’s leaves become prey to insects, birds, mammals, etc. Even after the plant has developed beyond the germination and sprouting stages it contains almost 50% of the levels of lectin found in the dry seeds. Approximately one third of this WGA is in the roots and two thirds is in the shoot, for at least 34 days [3]
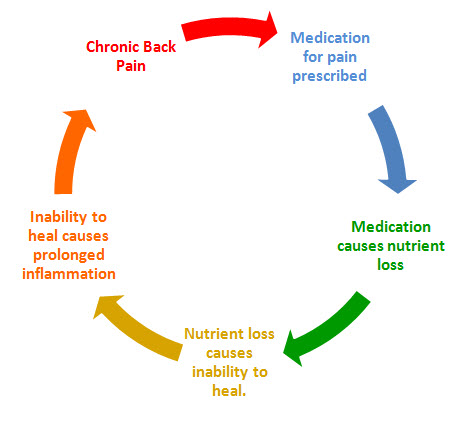
One way to gauge just how pervasive the adverse effects of WGA are among wheat-consuming populations is the popularity of the dietary supplement glucosamine. In the USA, a quarter billion dollars’ worth of the glucosamine is sold annually. The main source of glucosamine on the market is from the N-Acetylglucosamine rich chitin exoskelotons of crustaceans, like shrimp and crab. Glucosamine is used for reducing pain and inflammation. We do not have a dietary deficiency of the pulverized shells of dead sea critters, just as our use of NSAIDs is not caused by a deficiency of these synthetic chemicals in our diet. When we consume glucosamine supplements, the WGA, instead of binding to our tissues, binds to the pulverized chitin in the glucosamine supplements, sparing us from the full impact of WGA. Many millions of Americans who have greatly reduced their pain and suffering by ingesting glucosamine and NSAIDs may be better served by removing wheat, the underlying cause of their malaise, from their diets. This would result in even greater relief from pain and inflammation along with far less dependency on palliative supplements and medicines alike.
- WGA may be Pro-inflammatory
- At exceedingly small concentrations (nanomolar) WGA stimulates the synthesis of pro-inflammatory chemical messengers (cytokines) including Interleukin 1, Interleukin 6 and Interleukin 8 in intestinal and immune cells.[4]
- WGA has been shown to induce NADPH-Oxidase in human neutrophils associated with the “respiratory burst” that results in the release of inflammatory free radicals called reactive oxygen species[5] WGA has been shown to play a causative role in patients with chronic thin gut inflammation.[6]
- WGA may be Immunotoxic
- WGA induces thymus atrophy in rats[7] and may directly bind to, and activate, leukocytes [8].
- Anti-WGA antibodies in human sera have been shown to cross-react with other proteins, indicating that they may contribute to autoimmunity [9].
Indeed, WGA appears to play a role in the pathogenesis of celiac disease (CD) that is entirely distinct from that of gluten, due to significantly higher levels of IgG and IgA antibodies against WGA found in patients with CD, when compared with patients with other intestinal disorders. These antibodies have also shown not to cross-react with gluten antigens[10] [11]
- WGA may be Neurotoxic
- WGA can pass through the blood brain barrier (BBB) through a process called “adsorptive endocytosis”[12] and is able to travel freely among the tissues of the brain which is why it is used as a marker for tracing neural circuits[13]. WGA’s ability to pass through the BBB, pulling bound substances with it, has piqued the interest of pharmaceutical developers who are looking to find ways of delivering drugs to the brain. WGA has a unique binding affinity for N-Acetylneuraminic acid, a crucial component of neuronal membranes found in the brain, such as gangliosides which have diverse roles such as cell-to-cell contact, ion conductance, as receptors, and whose dysfunction has been implicated in neurodegenerative disorders. WGA may attach to the protective coating on the nerves known as the myelin sheath[14] and is capable of inhibiting nerve growth factor [15] which is important for the growth, maintenance, and survival of certain target neurons. WGA binds to N-Acetylglucosamine which is believed to function as an atypical neurotransmitter functioning in nocioceptive (pain) pathways.
- WGA may be Cytotoxic – WGA has been demonstrated to be cytotoxic to both normal and cancerous cell lines, capable of inducing either cell cycle arrest or programmed cell death (apoptosis). [16]
- WGA may interfere with Gene Expression – WGA demonstrates both mitogenic and anti-mitogenic[17] activities. WGA may prevent DNA replication[18] WGA binds to polysialic acid (involved in posttranslational modifications) and blocks chick tail bud development in embryogenesis, indicating that it may influence both genetic and epigenetic factors.
- WGA may disrupt Endocrine Function – WGA has also been shown to have an insulin-mimetic action, potentially contributing to weight gain and insulin resistance [19]. WGA has been implicated in obesity and “leptin resistance” by blocking the receptor in the hypothalamus for the appetite satiating hormone leptin. WGA stimulates epidermal growth factor which when upregulated is associated with increased risk of cancer. WGA has a particular affinity for thyroid tissue and has been shown to bind to both benign and malignant thyroid nodules[20] WGA interferes with the production of secretin from the pancreas, which can interfere with digestion and can cause pancreatic hypertrophy. WGA attaches to sperm and ovary cells, indicating it may adversely influence fertility.
- WGA may be Cardiotoxic
- WGA induces platelet activation and aggregration [21]. WGA has a potent, disruptive effect on platelet endothelial cell adhesion molecule-1, which plays a key role in tissue regeneration and safely removing neutrophils from our blood vessels.[22]
- WGA may adversely effect Gastrointestinal Function – WGA causes increased shedding of the intestinal brush border membrane, reduction in surface area, acceleration of cell losses and shortening of villi, via binding to the surface of the villi. WGA can mimic the effects of epidermal growth factor (EGF) at the cellular level, indicating that the crypt hyperplasia seen in celiac disease may be due to the growth-promoting effects of WGA. WGA causes cytoskeleton degradation in intestinal cells, contributing to cell death and increased turnover. WGA decreases levels of heat shock proteins in gut epithelial cells leaving these cells less well protected against the potentially harmful content of the gut lumen.[23]
- WGA may share pathogenic similarities with certain Viruses – There are a number of interesting similarities between WGA lectin and viruses. Both viral particles and WGA lectin are several orders of magnitude smaller than the cells they enter, and subsequent to their attachment to the cell membrane, are taken into the cell through a process of endocytosis. Both influenza and WGA gain entry through the sialic acid coatings of our mucous membranes (glycocalyx) each with a sialic acid specific substance, the neuriminidase enzyme for viruses and the sialic acid binding sites on the WGA lectin. Once the influenza virus and WGA lectin have made their way into wider circulation in the host body they are both capable of blurring the line in the host between self-and non-self. Influenza accomplishes this by incorporating itself into the genetic material of our cells and taking over the protein production machinery to make copies of itself, with the result that our immune system must attack its own virally transformed cell, in order to clear the infection. Studies done with herpes simplex virus have shown that WGA has the capacity to block viral infectivity through competitively binding to the same cell surface receptors, indicating that they may effect cells through very similar pathways. WGA has the capability of influencing the gene expression of certain cells, e.g. mitogenic/anti-mitogenic action, and like other lectins associated with autoimmunity, e.g. soy lectin, and viruses like Epstein-Barr Virus, WGA may be capable of causing certain cells to exhibit class 2 human leukocyte antigens (HLA-II), which mark them for autoimmune destruction by white blood cells. Since human antibodies to WGA have been shown to cross react with other proteins, even if WGA does not directly transform the phenotype of our cells into “other,” the resulting cross-reactivity of antibodies to WGA with our own cells would result in autoimmunity nonetheless.
It is my belief that a careful study of the wheat plant will reveal that, despite claims to the contrary, man does not have dominion over nature. All that he deems fit for his consumption may not be his inborn right. Though the wheat plant’s apparently defenseless disposition would seem to make it suitable for mass human consumption, it has been imbued with a multitude of invisible “thorns,” with WGA being its smallest and perhaps most potent defense against predation. While WGA may be an uninvited guest at our table, wheat is equally inhospitable to us. Perhaps the courteous thing to do, having realized our mistaken intrusion, is to lick our wounds and simply go our separate ways. Perhaps as the distance between man and his infatuation with wheat grows, he will grow closer to himself and will discover far more suitable forms of nourishment that Nature has not impregnated with such high levels of addictive and potentially debilitating proteins.
Although significant progress has been made in exposing the dark side of wheat over the past decade, gluten receives a disproportionate share of the attention. Given that modern bread wheat (Triticum Aestivum) is a hexaploid species containing three distinct sets of chromosomes capable of producing well over 23,000 unique proteins, it is not surprising that we are only now beginning to unravel the complexities of this plant’s many secrets. [1] What is unique about the WGA glycoprotein is that it can do direct damage to the majority of tissues in the human body without requiring a specific set of genetic susceptibilities and/or immune-mediated articulations. This may explain why chronic inflammatory and degenerative conditions are endemic to wheat-consuming populations even when overt allergies or intolerances to wheat gluten appear exceedingly rare. The future fate of wheat consumption, and by implication our health, may depend largely on whether or not the toxic qualities of WGA come to light in the general population.WGA lectin is an exceptionally tough adversary as it is formed by the same disulfide bonds that make vulcanized rubber and human hair so strong, flexible and durable. Like man-made pesticides, lectins are extremely small, resistant to break-down by living systems, and tend to accumulate and become incorporated into tissues where they interfere with normal biological processes. Indeed, WGA lectin is so powerful as an insecticide that biotech firms have used recombinant DNA technology to create genetically modified WGA-enhanced plants. We can only hope that these virtually unregulated biotech companies, who are in the business of playing God with the genetic infrastructure of Life, will realize the potential harm to humans that such genetic modifications can cause.It may strike some readers as highly suspect that wheat – the “staff of life” – which has garnered a reputation for “wholesome goodness” the world over, could contain a powerful health-disrupting anti-nutrient, which is only now coming to public attention. WGA has been overshadowed by the other proteins in wheat. Humans – not Nature – have spent thousands of years cultivating and selecting for larger and larger quantities of these proteins. These pharmacologically active, opiate-like proteins in gluten are known as gluten exorphins (A5, B4, B5, C) and gliadorphins. They may effectively anesthetize us, in the short term, to the long term, adverse effects of WGA. Gluten also contains exceptionally high levels of the excitotoxic l-aspartic and l-glutamic amino acids, which can also be highly addictive, not unlike their synthetic shadow molecules aspartame and monosodium glutamate.1 In a previous article on the topic, The Dark Side of Wheat: New Perspectives on Celiac Disease and Wheat Intolerance[2], we explored the role that these psychotropic qualities in grains played in ushering in civilization at the advent of the Neolithic transition 10,000 BC. No doubt the narcotic properties of wheat are the primary reason why suspicions about its toxicity have remained merely speculation for thousands upon thousands of years.Each grain contains about 1 microgram of WGA. That seems hardly enough to do any harm to animals our size. Lectins, however, are notoriously dangerous even in minute doses and can be fatal when inhaled or injected directly into the bloodstream. According to the U.S. Centers for Disease Control it takes only 500 micrograms (about half a grain of sand) of ricin (a lectin extracted from castor bean casings) to kill a human. A single, one ounce slice of wheat bread contains approximately 500 micrograms of WGA, which if it were refined to its pure form and injected directly into the blood, could, in theory, have platelet aggregating and erythrocyte agglutinizing effects strong enough to create an obstructive clot such as occurs in myocardial infarction and stroke. This, however, is not a likely route of exposure and in reality the immediate pathologies associated with lectins like ricin and WGA are largely restricted to the gastrointestinal tract where they cause mucosal injuries. The point is that WGA, even in small quantities, could have profoundly adverse effects, given suitable conditions. Ironically, WGA is exceptionally small, at 36 kilodaltons (approximately the mass of 36,000 hydrogen atoms) and it can pass through the cell membranes of the intestine with ease. The intestines will allow passage of molecules up to 1,000 kilodaltons in size. Moreover, one wheat kernel contains 16.7 trillion individual molecules of WGA, with each molecule of WGA having four N-Acetylglucosamine binding sites. The disruptive and damaging effects of whole wheat bread consumption are formidable in someone whose protective mucosal barrier has been compromised by something as simple as Non-Steroidal Anti-Inflammatory Drug (NSAID) use, or a recent viral or bacterial infection. The common consumption of both wheat and NSAIDs may suggest the frequency of the WGA vicious cycle. Anti-inflammatory medications, such as ibuprofen and aspirin, increase intestinal permeabilty and may cause absorption of even larger than normal quantities of pro-inflammatory WGA. Conversely, the inflammation caused by the absorption of WGA lectin is the very reason there is a great need for the inflammation-reducing effects of NSAIDs.To further underscore this point, the following are several ways that WGA depletes our health while glucosamine works against it:
- WGA may be Pro-inflammatory
- At exceedingly small concentrations (nanomolar) WGA stimulates the synthesis of pro-inflammatory chemical messengers (cytokines) including Interleukin 1, Interleukin 6 and Interleukin 8 in intestinal and immune cells.[4]
- WGA has been shown to induce NADPH-Oxidase in human neutrophils associated with the “respiratory burst” that results in the release of inflammatory free radicals called reactive oxygen species[5] WGA has been shown to play a causative role in patients with chronic thin gut inflammation.[6]
- WGA may be Immunotoxic
- WGA induces thymus atrophy in rats[7] and may directly bind to, and activate, leukocytes [8].
- Anti-WGA antibodies in human sera have been shown to cross-react with other proteins, indicating that they may contribute to autoimmunity [9].
Indeed, WGA appears to play a role in the pathogenesis of celiac disease (CD) that is entirely distinct from that of gluten, due to significantly higher levels of IgG and IgA antibodies against WGA found in patients with CD, when compared with patients with other intestinal disorders. These antibodies have also shown not to cross-react with gluten antigens[10] [11]
- WGA may be Neurotoxic
- WGA can pass through the blood brain barrier (BBB) through a process called “adsorptive endocytosis”[12] and is able to travel freely among the tissues of the brain which is why it is used as a marker for tracing neural circuits[13]. WGA’s ability to pass through the BBB, pulling bound substances with it, has piqued the interest of pharmaceutical developers who are looking to find ways of delivering drugs to the brain. WGA has a unique binding affinity for N-Acetylneuraminic acid, a crucial component of neuronal membranes found in the brain, such as gangliosides which have diverse roles such as cell-to-cell contact, ion conductance, as receptors, and whose dysfunction has been implicated in neurodegenerative disorders. WGA may attach to the protective coating on the nerves known as the myelin sheath[14] and is capable of inhibiting nerve growth factor [15] which is important for the growth, maintenance, and survival of certain target neurons. WGA binds to N-Acetylglucosamine which is believed to function as an atypical neurotransmitter functioning in nocioceptive (pain) pathways.
- WGA may be Cytotoxic – WGA has been demonstrated to be cytotoxic to both normal and cancerous cell lines, capable of inducing either cell cycle arrest or programmed cell death (apoptosis). [16]
- WGA may interfere with Gene Expression – WGA demonstrates both mitogenic and anti-mitogenic[17] activities. WGA may prevent DNA replication[18] WGA binds to polysialic acid (involved in posttranslational modifications) and blocks chick tail bud development in embryogenesis, indicating that it may influence both genetic and epigenetic factors.
- WGA may disrupt Endocrine Function – WGA has also been shown to have an insulin-mimetic action, potentially contributing to weight gain and insulin resistance [19]. WGA has been implicated in obesity and “leptin resistance” by blocking the receptor in the hypothalamus for the appetite satiating hormone leptin. WGA stimulates epidermal growth factor which when upregulated is associated with increased risk of cancer. WGA has a particular affinity for thyroid tissue and has been shown to bind to both benign and malignant thyroid nodules[20] WGA interferes with the production of secretin from the pancreas, which can interfere with digestion and can cause pancreatic hypertrophy. WGA attaches to sperm and ovary cells, indicating it may adversely influence fertility.
- WGA may be Cardiotoxic
- WGA induces platelet activation and aggregration [21]. WGA has a potent, disruptive effect on platelet endothelial cell adhesion molecule-1, which plays a key role in tissue regeneration and safely removing neutrophils from our blood vessels.[22]
- WGA may adversely effect Gastrointestinal Function – WGA causes increased shedding of the intestinal brush border membrane, reduction in surface area, acceleration of cell losses and shortening of villi, via binding to the surface of the villi. WGA can mimic the effects of epidermal growth factor (EGF) at the cellular level, indicating that the crypt hyperplasia seen in celiac disease may be due to the growth-promoting effects of WGA. WGA causes cytoskeleton degradation in intestinal cells, contributing to cell death and increased turnover. WGA decreases levels of heat shock proteins in gut epithelial cells leaving these cells less well protected against the potentially harmful content of the gut lumen.[23]
- WGA may share pathogenic similarities with certain Viruses – There are a number of interesting similarities between WGA lectin and viruses. Both viral particles and WGA lectin are several orders of magnitude smaller than the cells they enter, and subsequent to their attachment to the cell membrane, are taken into the cell through a process of endocytosis. Both influenza and WGA gain entry through the sialic acid coatings of our mucous membranes (glycocalyx) each with a sialic acid specific substance, the neuriminidase enzyme for viruses and the sialic acid binding sites on the WGA lectin. Once the influenza virus and WGA lectin have made their way into wider circulation in the host body they are both capable of blurring the line in the host between self-and non-self. Influenza accomplishes this by incorporating itself into the genetic material of our cells and taking over the protein production machinery to make copies of itself, with the result that our immune system must attack its own virally transformed cell, in order to clear the infection. Studies done with herpes simplex virus have shown that WGA has the capacity to block viral infectivity through competitively binding to the same cell surface receptors, indicating that they may effect cells through very similar pathways. WGA has the capability of influencing the gene expression of certain cells, e.g. mitogenic/anti-mitogenic action, and like other lectins associated with autoimmunity, e.g. soy lectin, and viruses like Epstein-Barr Virus, WGA may be capable of causing certain cells to exhibit class 2 human leukocyte antigens (HLA-II), which mark them for autoimmune destruction by white blood cells. Since human antibodies to WGA have been shown to cross react with other proteins, even if WGA does not directly transform the phenotype of our cells into “other,” the resulting cross-reactivity of antibodies to WGA with our own cells would result in autoimmunity nonetheless.
Given the multitude of ways in which WGA may disrupt our health, gain easy entry through our intestine into systemic circulation, and remain refractory to traditional antibody-based clinical diagnoses, it is altogether possible that the consumption of wheat is detracting from the general health of the wheat-consuming world and that we have been, for all these years, “digging our graves with our teeth.” This perspective may come as a great surprise to the health food industry whose particular love affair for whole wheat products has begun to go mass market. The increasingly hyped-up marketing of “whole wheat,” “sprouted grain,” and “wheat germ” enriched products, all of which may have considerably higher levels of WGA than their processed, fractionized, non-germinated and supposedly “less healthy” equivalents, may contribute to making us all significantly less healthy..[1] Desmond S. T. Nicholl, An Introduction to Genetic Engineering, 3rd Edition ISBN-13: 9780521615211[2] Ji, Sayer “The Dark Side of Wheat – New Perspectives on Celiac Disease & Wheat Intolerance.” Winter, 08’, Journal of Gluten Sensitivity[3] Distribution of Wheat Germ Agglutinin in Young Wheat Plants. Plant Physiol. 1980 Nov;66(5):950-955. PMID: 16661559[4] Effects of wheat germ agglutinin on human gastrointestinal epithelium: insights from an experimental model of immune/epithelial cell interaction. Toxicol and Applied Pharmacology 2009 Jun 1;237(2):146-53. Epub 2009 Mar 28. PMID 19332085[5] Wheat germ agglutinin induces NADPH-oxidase activity in human neutrophils by interaction with mobilizable receptors. Infection and Immunity. 1999 Jul;67(7):3461-8. PMID 10377127[6] Lectin glycosylation as a marker of thin gut inflammation. The FASEB Journal. 2008;22:898.3[7] Antinutritive effects of wheat-germ agglutinin and other N-acetylglucosamine-specific lectins.The British Journal of Nutrition 1993 Jul;70(1):313-21. PMID: 8399111[8] Lectinlike properties of pertussis toxin. . Infection and Immunity 1989 Jun;57(6):1854-7. PMID: 2722243[9] Natural human antibodies to dietary lectins. FEBS Lett. 1996 Nov 18;397(2-3):139-42. PMID: 8955334[10] Antibodies to wheat germ agglutinin in coeliac disease. Clin Exp Immunol. 1986 January; 63(1): 95–100. PMID: 3754186[11] Elevated levels of serum antibodies to the lectin wheat germ agglutinin in celiac children lend support to the gluten-lectin theory of celiac disease. Pediatr Allergy Immunol. 1995 May;6(2):98-102. PMID: 7581728[12] Transcytotic pathway for blood-borne protein through the blood-brain barrier. Proceedings from the National Academy of Sciences U S A. 1988 Jan;85(2):632-6. PMID: 2448779[13] Transsynaptic transport of wheat germ agglutinin expressed in a subset of type II taste cells of transgenic mice. BMC Neuroscience. 2008 Oct 2;9:96. PMID: 18831764[14] Distribution of concanavalin A and wheat germ agglutinin binding sites in the rat peripheral nerve fibres revealed by lectin/glycoprotein-gold histochemistry. The Histochem Journal. 1996 Jan;28(1):7-12. PMID: 8866643[15] Wheat germ agglutinin, concanavalin A, and lens culinalis agglutinin block the inhibitory effect of nerve growth factor on cell-free phosphorylation of Nsp100 in PC12h cells. Cell Struct and Function 1989 Feb;14(1):87-93. PMID:2720800[16] Wheat germ lectin induces G2/M arrest in mouse L929 fibroblasts. J Cell Biochem. 2004 Apr 15;91(6):1159-73. PMID: 15048871[17] Wheat germ agglutinin and concanavalin A inhibit the response of human fibroblasts to peptide growth factors by a post-receptor mechanism. J Cell Physiol. 1985 Sep;124(3):474-80. PMID: 2995421[18] DNA replication in cell-free extracts from Xenopus eggs is prevented by disrupting nuclear envelope function. J Cell Sci. 1992 Jan;101 ( Pt 1):43-53. PMID: 1569128[19] Effects of wheat germ agglutinin and concanavalin A on the accumulation of glycosaminoglycans in pericellular matrix of human dermal fibroblasts. A comparison with insulin. Acta Biochim Pol. 2001;48(2):563-72. PMID: 11732625[20] Analysis of lectin binding in benign and malignant thyroid nodules. Arch Pathol Lab Med. 1989 Feb;113(2):186-9. PMID: 2916907[21] Further characterization of wheat germ agglutinin interaction with human platelets: exposure of fibrinogen receptors. Thromb Haemost. 1986 Dec 15;56(3):323-7. PMID: 3105108[22] Wheat germ agglutinin-induced platelet activation via platelet endothelial cell adhesion molecule-1: involvement of rapid phospholipase C gamma 2 activation by Src family kinases. Biochemistry. 2001 Oct 30;40(43):12992-3001. PMID: 11669637[23] Decreased levels of heat shock proteins in gut epithelial cells after exposure to plant lectins. Gut. 2000 May;46(5):679-87. PMID: 10764712